Science
Galinpepimut-S: WT1 Targeting Immunotherapeutic
Our lead product candidate, galinpepimut-S (GPS), is a WT1-targeting cancer immunotherapeutic licensed from Memorial Sloan Kettering Cancer Center, which we are developing as a monotherapy and in combination with other therapeutic agents to treat different types of cancers.
The WT1 protein is one of the most widely expressed cancer proteins in multiple malignancies. In 2009, the National Cancer Institute ranked the WT1 protein as a top priority for immunotherapy.
WT1 is a protein that resides in the cell’s nucleus and participates in the process of cancer formation and progression. WT1 plays a key role in the development of the kidneys in fetal life, but then almost disappears from normal organs and tissues. In approximately 20 cancer types, WT1 becomes detectable again in at least 50% of tumor pathology specimens in the cells of these cancers. WT1 appears in large amounts, or becomes “overexpressed,” in numerous hematological malignancies, including acute myeloid leukemia (AML) and multiple myeloma (MM) as well as in many solid malignancies such as malignant pleural mesothelioma (MPM), ovarian cancer, and small cell lung cancer (SCLC).
Show more
GPS is a multi-peptide product that has been modified to enhance the degree and duration of the immune response against the WT1 protein. Two of the four peptides in the peptide mixture comprising GPS are deliberately mutated in a single amino acid residue. These mutated peptides are recognized by the immune system as non-self-entities and are therefore less likely to induce immune tolerance. These mutated peptides are designed using artificial intelligence (AI) to elicit strong T cell response against both mutated peptides and naturally occurring peptides in cancer cells. This concept is called the heteroclitic principle.
We believe that GPS targets malignancies and tumors characterized by an overexpression of the WT1 protein through direct activation of the patient’s immune system specifically and solely against the WT1 protein. A healthy immune system is designed to identify foreign or abnormal proteins expressed on tumor cells; however, this process is often defective in people with cancer. Typically, patients harboring WT1-positive malignancies have very few or no T cells specifically reactive or responsive to, and therefore activated by, WT1. T cells are involved in both sensing and killing abnormal cells, in addition to coordinating the activation of other cells in an immune response. T cells can be classified into two major subsets: CD4 cells and CD8 cells. CD8 cells, known as killer T cells, recognize, bind to, and kill target cells, including cancer cells. CD4 cells, known as helper T cells, are critical to providing the signals necessary for sustained CD8 cell responses and are also capable of direct anti-tumor activity.
Typically, cancer patients harboring WT1-positive malignancies have very few or no T cells specifically reactive or responsive to, and therefore activated by, WT1. GPS is designed to elicit both CD4 and CD8 cell immune responses. GPS is designed to activate CD8 cells which transform into cytotoxic T-lymphocytes (CTLs) that could attack and kill up to 10-20 WT1-positive cancer cells. Eventually, the CD4 cells could establish immunologic memory against a WT1-expressing cancer, which then are able to activate CTLs and B cells. These B-cells helped by the helper T cells produce antibodies to specific WT1 epitopes. This combination of actions initiates the process of immunity against WT1, potentially resulting in an anti-cancer effect.
We are currently developing GPS as a monotherapy and in combination with checkpoint inhibitors. For more information on our development program for GPS, please view our pipeline.
Show less
SLS009: Highly Selective CDK9 Inhibitor
SLS009 is a next generation highly selective cyclin-dependent kinase 9, or CDK9, inhibitor which we in-licensed from GenFleet Therapeutics (Shanghai), Inc. in March 2022. We have worldwide development and commercialization rights, except for Greater China.
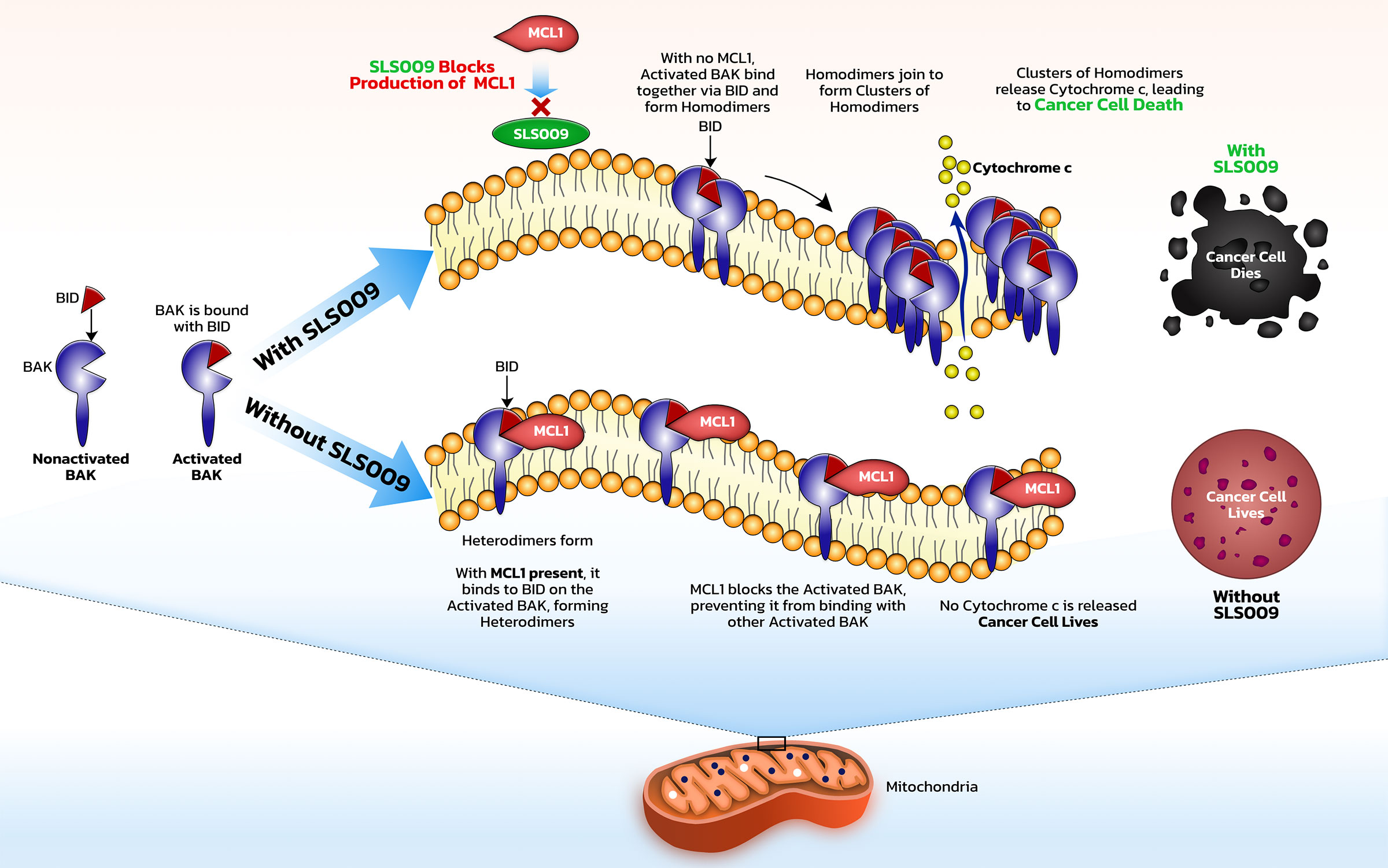
In general, CDKs are a family of protein kinases which are important in regulating cell processes. Deregulation of CDKs is associated with many cancers. CDK9 is one transcriptional subfamily and is a major cancer target whose activity has been shown to correlate negatively with overall survival in several cancer types, including hematologic cancers, such as acute myeloid leukemia and lymphomas, as well as solid cancers, such as pediatric soft tissue sarcomas and ovarian. CDK9, together with cyclin T1, forms positive transcription elongation factor b, or P-TEFb, which plays an important role in allowing long RNA strands to be quickly transcribed from DNA. P-TEFb is crucial for the synthesis of some of the key proteins, namely MYC, an oncogene, and MCL-1, a key anti-apoptotic protein, that drive growth of cancer cells and protect them from programmed cancer cell death. Inhibition of CDK9 can decrease the levels of MCL-1 and c-MYC which can result in apoptosis and cell cycle arrest.
It is important that other kinases are not inhibited along with CDK9. First generation CDK9 inhibitors worked across many CDK targets in addition to CDK9 and therefore had significant toxicity due to their low selectivity. Next generation CDK9 inhibitors, including SLS009, have the potential for higher specificity, potentially resulting in less toxicity and more consistent clinical activity.
In AML, some patients have leukemia sub-types that depend mostly on BCL2 and will be killed by venetoclax, a BCL2 inhibitor, while some will depend on MCL1 and will potentially be killed by SLS009. When patients respond to venetoclax, one of the primary drivers of their resistance is that the cells are no longer dependent on BCL2, but use other antiapoptotic methods to survive. MCL1 is a dominant mechanism of survival in patients that either don’t respond to a venetoclax-based regimen or respond then subsequently relapse. By harnessing an effect against MCL1 with SLS009, the main driver of cancer survival after venetoclax, SELLAS believes SLS009 could potentially be used synergistically with venetoclax to block the antiapoptotic effects of both MCL1 and BCL2.
For more information on our development of SLS009, visit our pipeline.
Publications
These scientific publications and presentations are provided for educational purposes only. They may discuss or mention SELLAS’ product candidates or therapeutic agents under clinical development, which have not been approved for marketing by any health or regulatory authority in any country.
- A randomized, open-label study of the efficacy and safety of galinpepimut-S (GPS) maintenance monotherapy compared to investigator’s choice of best available therapy (BAT) in patients with acute myeloid leukemia (AML) who have achieved complete remission (CR) after second-line salvage therapy. American Society of Clinical Oncology Annual Meeting (June 2-6, 2023)
- Maslak PG, et al. Phase 2 trial of a multivalent WT1 peptide vaccine (galinpepimut-S) in acute myeloid leukemia. Blood Adv. 2018;2:224-34. https://doi.org/10.1182/bloodadvances.2017014175
- Zauderer MG, et al. A randomized phase II trial of adjuvant galinpepimut-S, WT-1 analogue peptide vaccine, after multimodality therapy for patients with malignant pleural mesothelioma. Clin Cancer Res. 2017;23:7483-9; doi: https://doi.org/10.1158/1078-0432.ccr-17-2169
- Brayer J, et al. WT1 vaccination in AML and MDS: A pilot trial with synthetic analog peptides. Am J Hematol. 2015;90:602-7; doi: https://doi.org/10.1002/ajh.24014
- Maslak PG, et al. Vaccination with synthetic analog peptides derived from WT1 oncoprotein induces T-cell responses in patients with complete remission from acute myeloid leukemia. Blood. 2010;116:171-9; doi: https://doi.org/10.1182/blood-2009-10-250993
- Krug LM, et al. WT1 peptide vaccinations induce CD4 and CD8 T cell immune responses in patients with mesothelioma and non-small cell lung cancer. Cancer Immunol Immunother. 2010;59: 1467-79; doi: https://doi.org/10.1007/s00262-010-0871-8
- May RJ, et al. Peptide epitopes from the Wilms’ tumor 1 oncoprotein stimulate CD4+ and CD8+ T cells that recognize and kill human malignant mesothelioma tumor cells. Clin Cancer Res. 2007;13:4547-55; doi: https://doi.org/10.1158/1078-0432.ccr-07-0708
- Pinilla-Ibarz J, et al. Improved human T-cell responses against synthetic HLA-0201 analog peptides derived from the WT1 oncoprotein. Leukemia. 2006;20:2025-33; doi: https://doi.org/10.1038/sj.leu.2404380